Chromium-free leather choices
- Karl

- Mar 28, 2019
- 6 min read
It has long been the wish of leather product manufacturers and the leather industry, to be able to consistently produce leathers that do not contain chromium. Chromium tanning is dominant over most other tanning practices in most tanneries. Sure, there have been periods where the amount of chromium-free and vegetable tanned leather have increased but chromium tanning is still the main practice. In certain leather products, chromium-free is reserved for children or adults with skin sensitivities. Inherently the mood is that chromium-free is gaining traction but tends to decline when the price and effluent emission gets too overwhelming, or the physical properties do not meet specifications.
Glutaraldehyde
Since the 1950s, the industry has been looking for chromium alternatives. The development of glutaraldehyde tannage by the Eastern Regional Research Institute (United States Department of Agriculture, USDA) has provided a pre-tannage and chromium-free option to the industry for many years. Although chromium-free, glutaraldehyde is unsuitable for semi-processed leathers (shipped around the world) and is very poor for post-tanning chemical uptake. Glutaraldehyde is also biocidal and must be used carefully when being released into a biological effluent treatment plant. Many chromium-free leathers have low fastness properties and dull colours.
Glutaraldehyde is a 5-carbon molecule that has two aldehyde groups on either end, hence the reason it is often called glutardialdehyde. The aldehyde groups bind to lysine, asparagine and glutamine (if both still amidated), arginine, and sometimes histidine. Glutaraldehyde does not have the molecular size to be able to reach across from one collagen molecule to another, unless it undergoes a polymerisation reaction.
Polycarbamoyl Sulfonate (PCMS)
Some of the chromium-free patents that appear in the public domain had been lodged for many years, with their registration often appearing at a date where the tanning industry was not ready for alternatives to chromium. Polycarbamoyl sulfonate (PCMS) is one those.
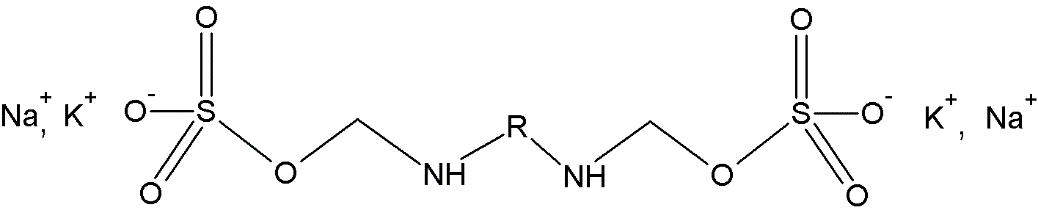
First developed in the early 1970s, PCMS is an anionic chemistry that is based on carbamates, see Figure 1. The anionic charge can alter, during reaction to the collagen, with sulfonate groups being lost. The carbamates themselves are reaction products resulting from isocyanate and water linkage (Reiners et al., 2013). They produce a pure white tannage without a significant pickle, and without a need for a basification step.
Figure 1. The simplified chemistry of the polycarbamoyl sulfonates.
The PCMS chemistry is simple, the affinity for the leather fibre is good, and the application is relatively straight forward. If dried out the tanned PCMS leathers will rehydrate relatively easily and the resulting leathers can be used in a wide variety of applications. The PCMS tannage, like most modern Cr-free tannages will struggle to penetrate thicker leathers. PCMS tannages are relatively non-ionic, so must be post-tanned with amphoteric products, or at the very least other cationic compounds.
Triazines/Diazines
Triazines are often used in the manufacture of reactive dyes. In a relatively short space of time the triazines, or diazines, have been modified and developed into truly amphoteric products that offer first generation opportunities as chromium alternatives (Reineking et al., 2012). The generalised chemistry structure is shown in Figure 2.
It can be seen, from Figure 2, that there are multiple places where R-groups can bind to the triazine/diazine structure. Some of the groups that link to the R1 and R2 positions could be a sulfonated phenolic structure or an alkyl chain that can provide lubricating or surfactant type properties.
Figure 2. The generalised structure of the modified diazines.
The group represented by the symbol Hal- denotes a halogen that is typically chlorine or fluorine. These groups are reported to be removed during the thermal treatment of the tannage. Failure to remove these groups will lead to them be of great concern for the tanner. The tertiary amine part of the structure can be protonated to provide a cationic site which makes the chemical uptake, by the leather more efficient than other chromium-free types.
The R3 group can also be a halogen, but it can be manufactured to give different functionality and reactivity. If R3 is a halogen then the compound will be very similar to the trichlorinated triazine/diazine dyes used in the 1970s. If R3 is not a halogen then the compound will be dichlorinated.
The use of the PCMS and diazine tannage has increased significantly across all sectors of the tanning sector, with the tannages even being used on exotic skins and hair-on. The tannages give clear white tannages and shave easily, allowing a convenient pre-tanning strategy. It is not unheard of in the industry to use these tannages as a first tan prior to shaving and a subsequent chromium tannage.
Glutaraldehyde, modified diazine, and PCMS tannage are not known for their production as semi-processed leathers. They do not transport well, and if the leathers dry out before post-tanning then there will be further problems going forward. The PCMS tannage does have the most favourable wetting back properties.
Buckyballs
Sodium aluminium silicate (NAS), has been sold in the tanning industry since the early 1990s. Sold as a zeolite, that was designed to improve the uptake of chromium, it could also act as a filler and the source of a pure white tannage. Concerns about metals, limited its application as consumers would often mistake aluminium as a heavy metal.
More recently, the misunderstandings about aluminium have been cleared up in the press and the general perception of aluminium has improved. Some automotive manufacturers, acknowledging the enormous abundance of aluminium in our soils, diet, clays, and glassware have begun to relax controls on aluminium. Figure 3 shows the typical structure of NAS and this “monomer” can often be folded into very elaborate structure that are used as wonderful images in science publications. These “buckyball” structures show complexity and size and illustrates why they are effective as filling agents.
Figure 3. The basic structure of sodium aluminium silicates.
As mentioned, the chemical structure of the modified diazines suggest that the molecule could be described as amphoteric (capable of positive and negative charge). The aluminium being a polyvalent cation will behave in a fashion very similar to chromium. Unlike aluminium sulfate, or aluminium chloride, the NAS has a higher resistance to hydrolysis at pH 4 – which makes it suitable in retanning as well.
Binding sites
The cationic charge of the modified diazine and the NAS introduces a renewed mindset that is difficult to find in other chromium-free technologies. It is difficult for chromium-free tanners to speak about high exhaustion. High exhaustion should always be at the heart of a committed passion to sustainability – as it focusses on the elimination of waste. For the less environmentally minded, high exhaustion still important for the elimination of waste. Chemicals that are taken up by the leathers are not disposed down the drain. Cationic functionality like that seen in the modified diazine, but particularly in the NAS technology, is possibly a step in the right direction to replace chromium tannage.
Figure 4. The exceptional fullness, cleanliness and roundness of correctly processed chromium-free.
The lack of focus, in the chromium-free arena, has resulted in approximately a 40-50% increase in the amount of chemicals used during post tannage. These large increases and poor uptake have resulted in greater burdens on effluent treatment plants and chemical costs. It should be a leading priority for tanneries to seek chromium-free tannages and ask for the following criteria:
High exhaustion of tanning and post-tanning chemicals (including dyes)
No restricted substances
Excellent rehydration properties when dried out
Transportation and storage as semi-processed material
Deep penetrating and effective tannage on split and unsplit material
Low cost
Renewable material
Summary
The current chromium-free choices fulfil only of a few of the criteria outlined above. As a result of this, they have often been described as first-generation products. Over time, we will see the emergence of the next generation products are set to be released. Leather conferences and journals have given some insights into what these products will look like.
Polyacrylate is a commonly used product in chromium-free retannage but has not made progress as a main tannage (Canudas, et al., 2019). Seemingly more emphasis will be placed on amphoteric products that allow better chemical fixation and more versatility, see Flowers (2018). TFL have also launched a masked zirconium product that gives a pure white retannage allowing an intense dyeing and better uptake of products (Döppert, 2018). Other cationic, metal-based materials will be a focus of research activity in the coming years.
It is exciting to see that the chemical industry is now producing a host of new products that are designed with their own purpose in mind, rather than old chromium-compatible technology that is being forced into chromium-free applications.
References
Heidemann, E. Fundamentals of leather manufacture. Roether, Darmstadt, Germany. 1993.
Covington, A.D. Tanning chemistry - the science of leather. RSC Publishing, Cambridge, UK. 2011.
Lanxess Germany, Gmbh. Compositions comprising of at least one compound containing a carbamoyl sulfonates and use of the same as tanning agent. Authors: Reiners, J., Tysoe, C., Wiechmann, J-D., Kruger, C., Grosch, R., Heinzelmann, F., Ebbinghaus, M., Kleban, M. United States Patent – US 2013/028806/A1. 8 Jul 2013.
Clariant International, Ltd. Non-metal tanning process. Authors: Reineking, C., Gamarino, R., Trimarco, L., Quaglierini, M., and Gisler, M. International Patent - WO 2012/055483/A1. 3 May 2012.
Canudas, M., Menna, N., Torrelles, A., De Pablo, J., and Morera, J.M. Polyacrylate ester-based polycarboxylate (PCE) as a new leather retanning agent. J. Am. Leather Chem. As. 114: 80-88. 2019.
Flowers, K. The role of acrylic amphoterics. International Leather Maker Sep/Oct. p. 112-114. 2018.
Döppert, F. In praise of zirconium. World Leather August/September: 28-29.











Comments